Discovering the most effective methodology to ship chemotherapeutic medication to tumor cells will be difficult. Ideally, the therapies goal tumor cells whereas leaving wholesome cells alone.
Immunoliposomes may very well be the reply. They’ll effectively bind to antigens on tumor cell surfaces by way of their surface-targeting ligands, permitting tumor cells adequate time to take up the “poison.” The advantages of immunoliposomes in most cancers remedy have been extensively documented within the final 4 a long time. Nonetheless, immunoliposomal medication haven’t but made it to the market, though they’ve been demonstrated in laboratories since 1981.
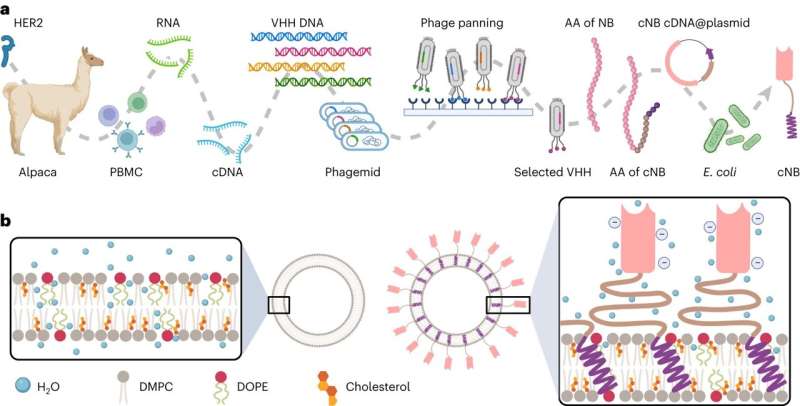
Why? One key barrier is the shortage of a large-scale, low-cost, but possible manufacturing approach. Grafting concentrating on ligands onto plain liposomes to type immunoliposomes includes about half a dozen steps and might result in potential issues.
Yuan Wan, an affiliate professor at Binghamton College’s Thomas J. Watson Faculty of Engineering and Utilized Science, lately published research in the journal Nature Nanotechnology outlining a one-step manufacturing course of for immunoliposome manufacturing. It doesn’t require any chemical conjugation and related chemical reagents, making it eco-friendly.
“The standard manufacturing means of immunoliposomes is comparatively complicated,” stated Wan, a school member within the Division of Biomedical Engineering. “It includes a number of chemical conjugation and purification. Chemical conjugation and required reagents impair the steadiness and antigen binding of concentrating on ligands. The multistep course of results in payload leakage and product loss.”
“So, immunoliposomes are much less engaging to industrial producers due to their low yield, excessive manufacturing prices, and the excessive danger of batch-to-batch variation. These shortcomings hinder business manufacturing and scientific use of immunoliposomes.”
What makes Wan’s analysis totally different is the addition of engineered chimeric nanobodies, which have a “sticky” finish. Greater than 2,500 nanobodies can combine on the surface of a single 100-nanometer liposome, which is about 1,000 occasions smaller than a human hair.
This methodology is less complicated, quicker and cheaper than conventional strategies, and it permits for extra management over the ultimate product. The floor nanobodies additionally type a protecting layer across the liposome, which could assist it keep away from being cleared by the physique too shortly and permit it to remain within the bloodstream longer.
One other huge benefit is that this methodology would not require harsh chemical substances. Conventional strategies typically use a substance known as polyethylene glycol (PEG), which typically could cause issues for sufferers, even loss of life. Due to these considerations, the federal Meals and Drug Administration requires further monitoring for medication containing PEG.
“One thing actually attention-grabbing we discovered is that when these chimeric nanobodies are inserted into the lipid bilayer, they really enhance the rigidity and thermal stability of the whole immunoliposomes. So, the medication packed inside can maintain up for an excellent 10 months with out apparent leaks,” Wan stated.
As a result of there are additionally round 20 plain liposomal medication already in use, Wan is hopeful that—with additional analysis and medical trials—immunoliposomes may very well be manufactured and get federal approval for scientific use.
“We’re additionally engaged on growing new chimeric nanobodies to ramp up manufacturing by not less than 30 occasions. It would make the manufacturing value of those chimeric nanobodies a lot decrease.” Wan stated.
Extra data:
Md. Mofizur Rahman et al, Chimeric nanobody-decorated liposomes by self-assembly, Nature Nanotechnology (2024). DOI: 10.1038/s41565-024-01620-6
Supplied by
Binghamton University
Quotation:
Chimeric nanobody analysis seems to enhance chemotherapy drug supply (2024, March 12)
retrieved 12 March 2024
from https://phys.org/information/2024-03-chimeric-nanobody-chemotherapy-drug-delivery.html
This doc is topic to copyright. Aside from any honest dealing for the aim of personal research or analysis, no
half could also be reproduced with out the written permission. The content material is offered for data functions solely.







