Oxide ion conductors utilized in solid-state gasoline cells usually fail to succeed in full potential when working at temperatures beneath 500 oC, however researchers from Tokyo Tech have not too long ago discovered an answer to this drawback. They demonstrated excessive conductivity and stability in bismuth-containing Sillén oxyhalides with triple fluorite-like layers (e.g., 10 mS/cm at 431 oC; 204 instances greater conductivity than that of typical conductors at 310 oC).
Strong oxide gasoline cells (SOFCs) are recognized for his or her high efficiency and improved security in comparison with different kinds of gasoline cells. One of many key elements in SOFC efficiency is the solid electrolyte: the oxide ion conductor. Its glorious electrochemical properties make it a really perfect electrolyte not just for SOFC functions but in addition for stable oxide electrolyzer cells (SOECs), sensors, and oxygen separation membranes.
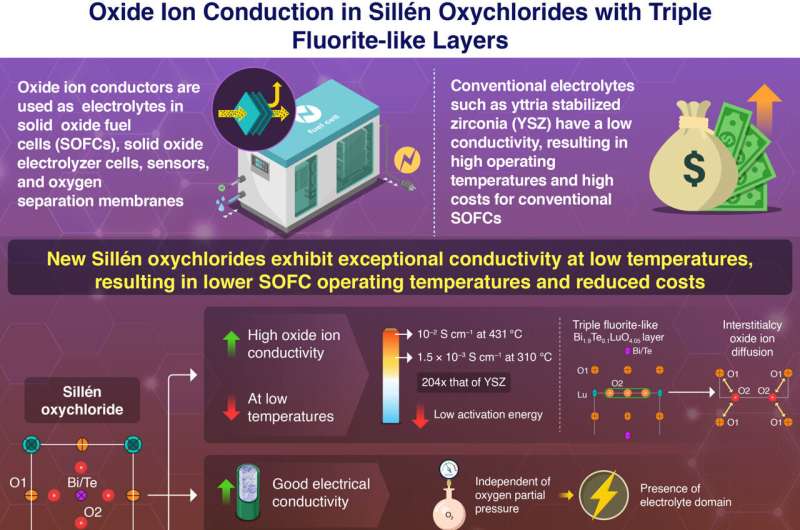
Regardless of the numerous benefits of oxide ion conductors, generally used oxide ion conductors similar to yttria-stabilized zirconia (YSZ) require extraordinarily excessive working temperatures of 1000−700 oC. Over lengthy intervals, such excessive temperatures may be detrimental to SOFC efficiency. To forestall degradation, costly heat-resistant alloys are used, which robotically will increase the manufacturing price of SOFCs. As well as, there’s a lack of steady oxide ion conductors that exhibit a conductivity of 10−2 S cm−1 beneath 500 oC.
To bridge the prevailing hole within the steady oxide ion electrolytes with decrease working temperatures, a workforce of researchers from the Tokyo Institute of Technology (Tokyo Tech), led by Professor Masatomo Yashima, not too long ago turned their consideration to Sillén oxyhalides.
Within the newest Journal of the American Chemical Society study, they synthesized a collection of bismuth (Bi)-containing Sillén oxyhalides and investigated their electrical and structural properties. “We selected supplies containing Bi species as a result of they’re recognized to exhibit excessive oxide ion conductivity. Moreover, the mum or dad supplies Sillén oxyhalides possess triple fluorite-like layers with interstitial oxygen websites, which might result in interstitialcy oxide ion diffusion,” says Yashima.
On this examine, the researchers synthesized Sillén oxychlorides with the molecular method Bi2−xTexLuO4+x/2Cl (x = 0, 0.1, and 0.2).
The workforce’s experiments on Bi1.9Te0.1LuO4.05Cl confirmed excessive bulk conductivity of 10 mS/cm (= 0.01 Ω−1 cm−1), which is an ordinary for sensible use in gasoline cells, at a a lot decrease temperature of 431 oC than 644 oC of the standard materials YSZ (Yttria-Stabilized Zirconia). Bi1.9Te0.1LuO4.05Cl additionally reveals excessive bulk conductivity of 1.5 × 10−3 S cm−1 at a low temperature of 310 °C, which is greater than 200 instances greater than that of YSZ.
This conduct was attributed to the low activation vitality. Additional evaluation utilizing neutron diffraction experiments, DFT calculations, and molecular dynamics simulations revealed that the low activation vitality and excessive conductivity emerge because of the presence of interstitialcy oxide ion diffusion within the triple fluorite-like layer of Sillén oxychlorides.
Other than glorious oxide ion conductivity, Bi1.9Te0.1LuO4.05Cl additionally exhibited excessive electrical conductivity unbiased of the oxygen partial stress at 431 °C, which indicated the presence of an electrolyte area. It additionally maintained excessive chemical stability below CO2 stream at 400 °C and ambient air at 600 and 400 °C.
The outcomes of this examine demonstrated that triple fluorite-like layers of Sillén oxyhalides can be utilized to develop excessive ionic conductors at temperatures beneath 500 °C, fixing a long-standing drawback with oxide ion conductors. The excessive ionic and electrical conductivity and chemical stability of Bi1.9Te0.1LuO4.05Cl might open new avenues for the event of superior oxide ion conductors with a triple fluorite-like layer.
“Strong oxide gasoline cells are being extensively accepted because the new-age vitality supply, however the associated fee is excessive as a result of their excessive working temperature. By this analysis, we have now developed new oxide ion conductors, which might considerably decrease the operating temperature and cut back their price,” concludes Yashima.
Extra data:
Nachi Ueno et al, Excessive Conductivity and Diffusion Mechanism of Oxide Ions in Triple Fluorite-Like Layers of Oxyhalides, Journal of the American Chemical Society (2024). DOI: 10.1021/jacs.4c00265
Quotation:
Distinctive oxide ion conductivity at decrease temperatures provides potential resolution for solid-state gasoline cells (2024, April 10)
retrieved 10 April 2024
from https://techxplore.com/information/2024-04-exceptional-oxide-ion-temperatures-potential.html
This doc is topic to copyright. Other than any honest dealing for the aim of personal examine or analysis, no
half could also be reproduced with out the written permission. The content material is supplied for data functions solely.







