MIT chemical engineers have devised an environment friendly technique to convert carbon dioxide to carbon monoxide, a chemical precursor that can be utilized to generate helpful compounds similar to ethanol and different fuels.
If scaled up for industrial use, this course of might assist to take away carbon dioxide from power plants and different sources, decreasing the quantity of greenhouse gases which might be launched into the ambiance.
“This could can help you take carbon dioxide from emissions or dissolved within the ocean, and convert it into worthwhile chemical substances. It is actually a path ahead for decarbonization as a result of we are able to take CO2, which is a greenhouse gasoline, and switch it into issues which might be helpful for chemical manufacture,” says Ariel Furst, the Paul M. Prepare dinner Profession Improvement Assistant Professor of Chemical Engineering and the senior writer of the examine.
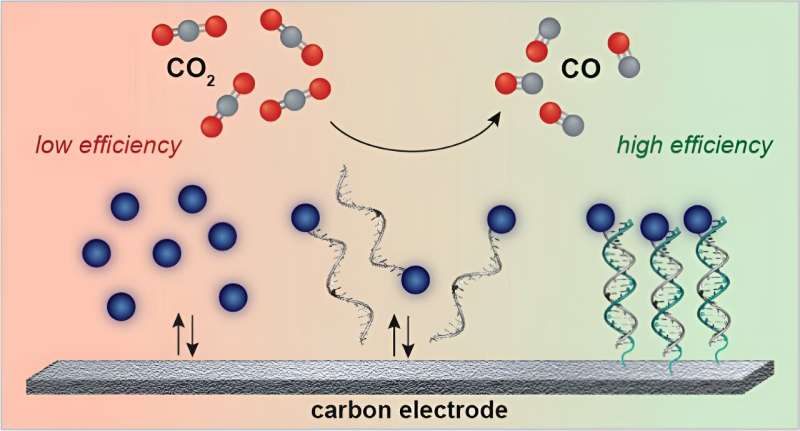
The brand new method makes use of electrical energy to carry out the chemical conversion, with assist from a catalyst that’s tethered to the electrode floor by strands of DNA. This DNA acts like Velcro to maintain all of the response elements in shut proximity, making the response rather more environment friendly than if all of the elements had been floating in resolution.
Furst has began an organization known as Helix Carbon to additional develop the expertise. Former MIT postdoc Gang Fan is the lead writer of the paper, which is printed within the Journal of the American Chemical Society Au. Different authors embody Nathan Corbin Ph.D. ’21, Minju Chung Ph.D. ’23, former MIT postdocs Thomas Gill and Amruta Karbelkar, and Evan Moore ’23.
Breaking down CO2
Changing carbon dioxide into helpful merchandise requires first turning it into carbon monoxide. A method to do that is with electrical energy, however the quantity of power required for that kind of electrocatalysis is prohibitively costly.
To attempt to deliver down these prices, researchers have tried utilizing electrocatalysts, which may pace up the response and scale back the quantity of power that must be added to the system. One kind of catalyst used for this response is a category of molecules generally known as porphyrins, which comprise metals similar to iron or cobalt and are related in construction to the heme molecules that carry oxygen in blood.
Throughout any such electrochemical response, carbon dioxide is dissolved in water inside an electrochemical gadget, which accommodates an electrode that drives the response. The catalysts are additionally suspended within the resolution. Nevertheless, this setup is not very environment friendly as a result of the carbon dioxide and the catalysts have to encounter one another on the electrode floor, which does not occur fairly often.
To make the response happen extra ceaselessly, which might increase the effectivity of the electrochemical conversion, Furst started engaged on methods to connect the catalysts to the floor of the electrode. DNA gave the impression to be the best alternative for this utility.
“DNA is comparatively cheap, you’ll be able to modify it chemically, and you’ll management the interplay between two strands by altering the sequences,” she says. “It is like a sequence-specific Velcro that has very sturdy however reversible interactions that you could management.”
To connect single strands of DNA to a carbon electrode, the researchers used two “chemical handles,” one on the DNA and one on the electrode. These handles might be snapped collectively, forming a everlasting bond. A complementary DNA sequence is then connected to the porphyrin catalyst, in order that when the catalyst is added to the answer, it would bind reversibly to the DNA that is already connected to the electrode—similar to Velcro.
As soon as this method is ready up, the researchers apply a possible (or bias) to the electrode, and the catalyst makes use of this power to transform carbon dioxide within the resolution into carbon monoxide. The response additionally generates a small quantity of hydrogen gasoline, from the water. After the catalysts put on out, they are often launched from the floor by heating the system to interrupt the reversible bonds between the 2 DNA strands, and changed with new ones.
An environment friendly response
Utilizing this method, the researchers had been in a position to increase the Faradaic effectivity of the response to 100%, which means that the entire electrical energy that goes into the system goes immediately into the chemical reactions, with no power wasted. When the catalysts usually are not tethered by DNA, the Faradaic effectivity is barely about 40%.
This expertise might be scaled up for industrial use pretty simply, Furst says, as a result of the carbon electrodes the researchers used are a lot cheaper than standard metallic electrodes. The catalysts are additionally cheap, as they do not comprise any precious metals, and solely a small focus of the catalyst is required on the electrode floor.
By swapping in several catalysts, the researchers plan to strive making different merchandise similar to methanol and ethanol utilizing this method. Helix Carbon, the corporate began by Furst, can be engaged on additional growing the expertise for potential industrial use.
Extra info:
Gang Fan et al, Extremely Environment friendly Carbon Dioxide Electroreduction through DNA-Directed Catalyst Immobilization, JACS Au (2024). DOI: 10.1021/jacsau.3c00823
This story is republished courtesy of MIT Information (web.mit.edu/newsoffice/), a preferred website that covers information about MIT analysis, innovation and educating.
Quotation:
Engineers discover a new technique to convert carbon dioxide into helpful merchandise (2024, March 27)
retrieved 28 March 2024
from https://techxplore.com/information/2024-03-carbon-dioxide-products.html
This doc is topic to copyright. Other than any honest dealing for the aim of personal examine or analysis, no
half could also be reproduced with out the written permission. The content material is offered for info functions solely.







