Glycerol, a serious by-product of biomass refining accounting for about 10% of the yield, presents a big problem as a result of its international surplus. The presence of a number of energetic hydroxyl teams in glycerol unveils huge potential for the manufacturing of high-value chemical substances. Formic acid (FA), a key product of glycerol conversion, is a vital natural chemical uncooked materials with excessive demand in sectors comparable to pesticides, prescription drugs, and power.
Oxidizing glycerol to FA not solely mitigates the waste brought on by useful resource surplus but additionally caters to the long run wants of FA gas cells. At present, the commercial manufacturing of FA primarily depends on methanol derived from petroleum and natural gas, making the electrocatalytic conversion of biomass-based glycerol into FA extremely promising.
Nevertheless, the electrocatalytic oxidation of glycerol response (RGOR) is complicated, involving dehydrogenation, adsorption/desorption, and C-C bond breaking of response intermediates, posing challenges to the response’s effectivity and selectivity.
Just lately, a analysis group led by Prof. Kai Yan of Solar Yat-sen College, China, using a mix of density practical principle (DFT) calculations and experimental strategies, has unveiled the essential position of the energetic species OH* within the EGOR course of in producing FA. DFT evaluation, ranging from a thermodynamic perspective, investigated the mechanism of OH* within the EGOR course of.
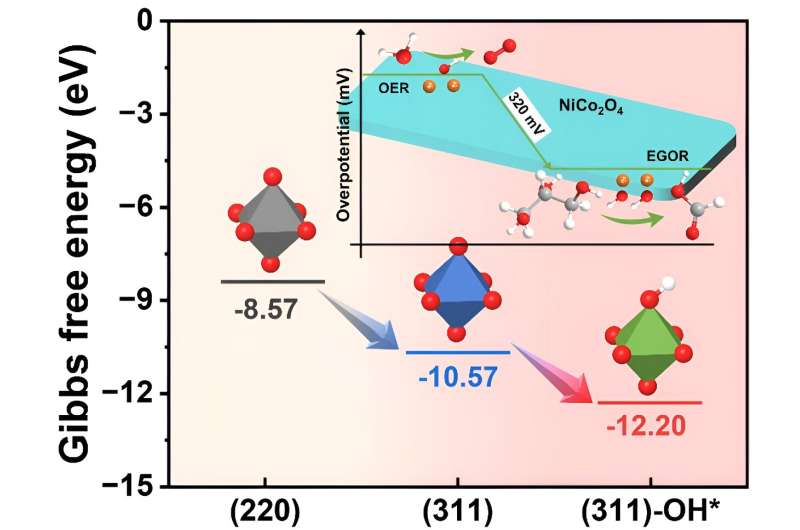
It was discovered that floor OH* species, by lowering the adsorption power of glycerol on the NiCo2O4 catalyst surface (from -12.20 to -10.57 eV), facilitated the EGOR course of and optimized the rate-determining step (RDS) by altering the adsorption power of intermediates, shifting from the much less environment friendly dehydrogenation of glyceric acid to the extra environment friendly dehydrogenation step of glyceraldehyde.
Furthermore, the adsorption power of OH* in the course of the EGOR course of was considerably decrease in comparison with the oxygen evolution response (OER) course of (0.66 vs. 2.70 eV), indicating the preferential incidence of EGOR over OER.
Additional, the efficiency of a meticulously designed NiCo2O4 electrode in EGOR was investigated by means of electrochemical strategies. In a combined electrolyte of 1 mol L-1 KOH and 0.1 mol L-1 glycerol, the onset potential of the electrode dropped to 1.16 VRHE, considerably outperforming OER. Rotating ring-disk electrode (RRDE) experiments additionally confirmed the preferential incidence of EGOR, aligning with the DFT evaluation findings.
Based mostly on the traditional proton-coupled electron switch mechanism, two doable electrochemical oxidation pathways (direct oxidation pathway and oblique oxidation pathway) of OH* have been investigated by utilizing multi-step potentiation and simultaneous electron resonance strategies. Experimental outcomes demonstrated that the distinctive efficiency of the NiCo2O4 electrode in EGOR is carefully associated to the in-situ technology of OH* straight taking part within the response.
In a long-term cycle stability take a look at of 120 h, the catalyst additionally confirmed an environment friendly and secure glycerol conversion fee (89%) and formic acid selectivity (70%). This work gives useful steerage and insights for the design and growth of environment friendly and secure catalysts for glycerol oxidation. The outcomes had been printed in Chinese Journal of Catalysis.
Extra data:
Yan Duan et al, Integration of principle prediction and experimental electrooxidation of glycerol on NiCo2O4 nanosheets, Chinese language Journal of Catalysis (2024). DOI: 10.1016/S1872-2067(23)64585-1
Supplied by
Chinese Academy of Sciences
Quotation:
Integration of principle prediction and experimental electrooxidation of glycerol on nanosheets (2024, March 25)
retrieved 25 March 2024
from https://phys.org/information/2024-03-theory-experimental-electrooxidation-glycerol-nanosheets.html
This doc is topic to copyright. Aside from any honest dealing for the aim of personal research or analysis, no
half could also be reproduced with out the written permission. The content material is offered for data functions solely.







