Carbon nanotubes have proven promise for all the things from microelectronics to aviation to power storage. Researchers assume this materials would possibly someday fulfill the science fiction dream of making an elevator to area.
So why aren’t they used extra typically?
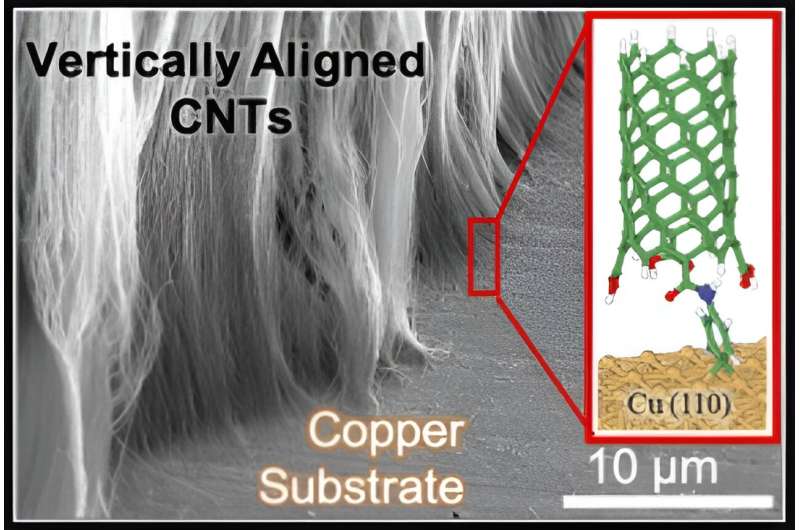
College of Cincinnati chemist Noe Alvarez stated one impediment has been the irritating incapability to hyperlink carbon nanotubes to metallic surfaces in a strong connection for sensors, transistors and different makes use of. These hole tubes have a diameter of only a billionth of a meter however will be many centimeters lengthy.
“We would like our experiments to be reproducible and constant, however that is not simply doable with nanotubes as a result of we will not management how properly they’re related to metallic surfaces,” he stated.
However he and his collaborators have demonstrated a brand new chemical course of that grafts nanotubes to metallic surfaces to create a robust, constant, conductive hyperlink. The study was revealed within the journal Nanoscale Advances.
In previous iterations, carbon nanotubes have been dispersed in an answer to make what Alvarez likens to “moist spaghetti” that sticks to a metal surface.
“However there isn’t a strong connection. Nothing is basically holding the nanotubes to the floor,” he stated.
So measurements of properties similar to electrical conductivity have been imprecise and inconsistent.
Alvarez and his analysis companions at Texas A&M College, led by chemical engineering Professor Jorge Seminario, demonstrated methods to bond nanotubes chemically to copper, aluminum, gold and different metallic surfaces.
Alvarez and his collaborators acquired a $720,000 grant from the Nationwide Science Basis to elaborate on their chemical discovery within the subsequent three years.
“Why do not we see carbon nanotubes in widespread industrial functions regardless that they’ve a lot potential? We have now so much to determine,” UC doctoral pupil and examine lead writer Chaminda Nawarathne stated.
Alvarez and his co-authors found by means of computational calculations that carbon atoms within the natural hyperlink really bond with two copper atoms, creating an particularly robust bond.
“That explains why our nanotubes as soon as they’re chemically related keep related,” Alvarez stated.
Carbon nanotubes are notoriously robust molecules. Their molecular construction creates a chic hexagonal lattice. “Carbon bonds are the strongest bonds. They’re covalent bonds. That is why diamond is the toughest materials as a result of they’re carbon-carbon bonds,” Alvarez stated.
Whereas carbon atoms in diamonds are single bonds, carbon nanotubes have conjugated double-bonded atoms, making them even stronger than diamonds.
Cables created from robust however light-weight carbon nanotubes have been envisioned for creating “area elevators” that would ferry tools into orbit, Alvarez stated. A space elevator was depicted within the opening scene of the Brad Pitt film “Advert Astra.”
However power is only one of their unique properties.
Carbon nanotubes are used to create the blackest artificial materials on Earth. Alvarez stated their robust bonds with metallic may result in higher paints and coatings.
“Nanotubes are pretty inert. They’re very steady. You possibly can conjugate them with out breaking their bonds. Semiconducting nanotubes even have fluorescence properties—they’ll generate gentle,” Alvarez stated. “So the listing of functions goes on and on.”
Nawarathne stated he’s pursuing potential functions in power storage.
“Now that we are able to bond the carbon nanotubes to a present collector or metallic probe, we are able to make very steady electrodes for supercapacitors,” Nawarathne stated.
UC chemistry college students “develop” nanotubes on silicon disks utilizing a course of known as catalytic chemical vapor deposition in tools that heats reagents and an iron catalyst to 1,450°F.
“It is red-hot,” Alvarez stated, pointing to an object seen by means of a glass window within the oven-sized machine. “That is like a baking pan. The catalyst goes in right here.”
After 45 minutes, a skinny layer of carbon nanotubes seems on the silicon. From there, researchers have been capable of electrograft the nanotubes onto a wide range of metallic surfaces. Initially, they used bundles of nanotubes however with refined processes realized they’ll join vertically aligned nanotubes.
“It is like making an attempt to attach wool again to a sheep. You’ve gotten yarn that has been sheared from the sheep. We’re capable of join particular person fibers again to the sheep chemically,” he stated.
Extra info:
Chaminda P. Nawarathne et al, Creating covalent bonds between Cu and C on the interface of metallic/open-ended carbon nanotubes, Nanoscale Advances (2023). DOI: 10.1039/D3NA00500C
Supplied by
University of Cincinnati
Quotation:
Researchers uncover option to bind nanotubes to metals (2024, February 29)
retrieved 29 February 2024
from https://phys.org/information/2024-02-nanotubes-metals.html
This doc is topic to copyright. Aside from any truthful dealing for the aim of personal examine or analysis, no
half could also be reproduced with out the written permission. The content material is offered for info functions solely.







